Osmosis is the chemical-physical phenomenon whereby two liquid solutions spread into one another through a semipermeable membrane and is caused by the difference in concentration between the two liquids.
The “osmotic cements” are cementitious waterproofing products developed in Germany at the end of the 70s, based on an “osmotic model” of interpretation of the hydration and solidification processes of hydraulic binders.
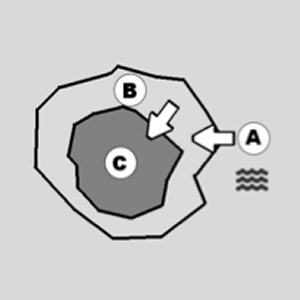
The partially anhydrous cement granule (C) represents the “concentrated solution; the “cement gel” (B) is the semipermeable membrane; while water (A) is the diluted solution.
In the described context, the heat of hydration drastically reduces the efficiency and duration of the hydration process: the cement granules are only “partially hydrated”. The experiments carried out had in fact highlighted the particular ability of tartaric acid derivatives to delay and balance the hydration processes, facilitating osmotic exchanges, gel stabilization and crystalline transition with the result of deeper and more complete hydration. The better “balance” of the osmotic phase, made possible above all by catalysts based on derivatives of tartaric acid, resulted in significant increases in quality and performance, both in terms of diffusion, adherence and intrinsic impermeability of the binder matrix.
THE CEMENTITIOUS WATER BARRIER,
DIFFUSIVE AND WATERPROOF
The experiments carried out had shown that specially formulated cement systems, as concrete translations of the described acquisitions, initially defined as “osmotic cements” , provided important waterproofing performances both in the occasional and permanent presence of rainwater, natural water, groundwater, etc.
The particular adhesion of these systems, determined by the deep osmotic diffusion, also made them suitable for operating both in conditions of direct thrust (positive thrust) and negative (counter thrust).